Ketamine troches are an emerging form of ketamine administration used in the treatment of various mental health disorders, including depression, anxiety, and PTSD. These lozenge-like tablets offer a unique, patient-friendly alternative to traditional intravenous (IV) or intranasal ketamine treatments. However, as with any compounded medication, there are several challenges related to maintaining consistency, quality, and adherence to compounding standards. In this article, we will explore the compounding standards for ketamine troches, the quality control processes involved, and the consistency challenges that must be addressed to ensure safety and efficacy.
Understanding Ketamine Troches: A Modern Approach to Treatment
Ketamine, a drug initially used as an anesthetic, has gained recognition for its rapid antidepressant effects. It is often used in patients with treatment-resistant depression, where traditional medications have failed. While ketamine is most commonly administered intravenously or intranasally in clinical settings, ketamine troches provide an oral, dissolvable option that can be taken at home. This method has opened up new possibilities for patients who may prefer a more comfortable and accessible treatment option.
Unlike traditional oral medications, ketamine troches are compounded in pharmacies to specific patient needs. This personalized approach ensures that the dosage is tailored to the individual’s treatment requirements. While troches can be an excellent option for many patients, there are significant challenges in maintaining consistent quality and ensuring that each dose is safe and effective.
Compounding Standards for Ketamine Troches
The preparation of ketamine troches falls under strict compounding regulations, as they are not commercially available in this form. Compounding pharmacies must adhere to standards set by organizations such as the United States Pharmacopeia (USP) and the U.S. Food and Drug Administration (FDA) to ensure the drugs are produced safely.
The compounding of ketamine troches typically involves pharmacists mixing the active ketamine ingredient with excipients, including flavoring agents and binders, to create a pleasant-tasting, dissolvable tablet. These tablets are then dispensed in individualized doses as prescribed by a healthcare provider. While the process allows for customization, it also introduces risks if the compounding standards are not strictly followed.
Key compounding standards include:
- Ingredient Quality: The quality of the ketamine used in compounding is paramount. Pharmacies must source pharmaceutical-grade ketamine to ensure that the active ingredient is pure and uncontaminated.
- Accurate Dosage: Achieving the correct ketamine dose in each troche is critical for patient safety. Compounding pharmacies use specialized equipment to weigh and mix ingredients accurately. Failure to do so can lead to inconsistent dosing, resulting in either suboptimal treatment or potential overdose.
- Sterility: While ketamine troches are taken orally, they still need to be prepared in sterile conditions to avoid contamination. Maintaining clean environments and adhering to sterilization procedures is crucial during the compounding process.
- Labeling and Documentation: Proper labeling and clear documentation are essential to ensure that the final product is correctly identified, dispensed, and tracked for potential recalls or safety concerns. This includes providing detailed information about the dosage, ingredients, and instructions for use.
Quality Control in Compounding Ketamine Troches
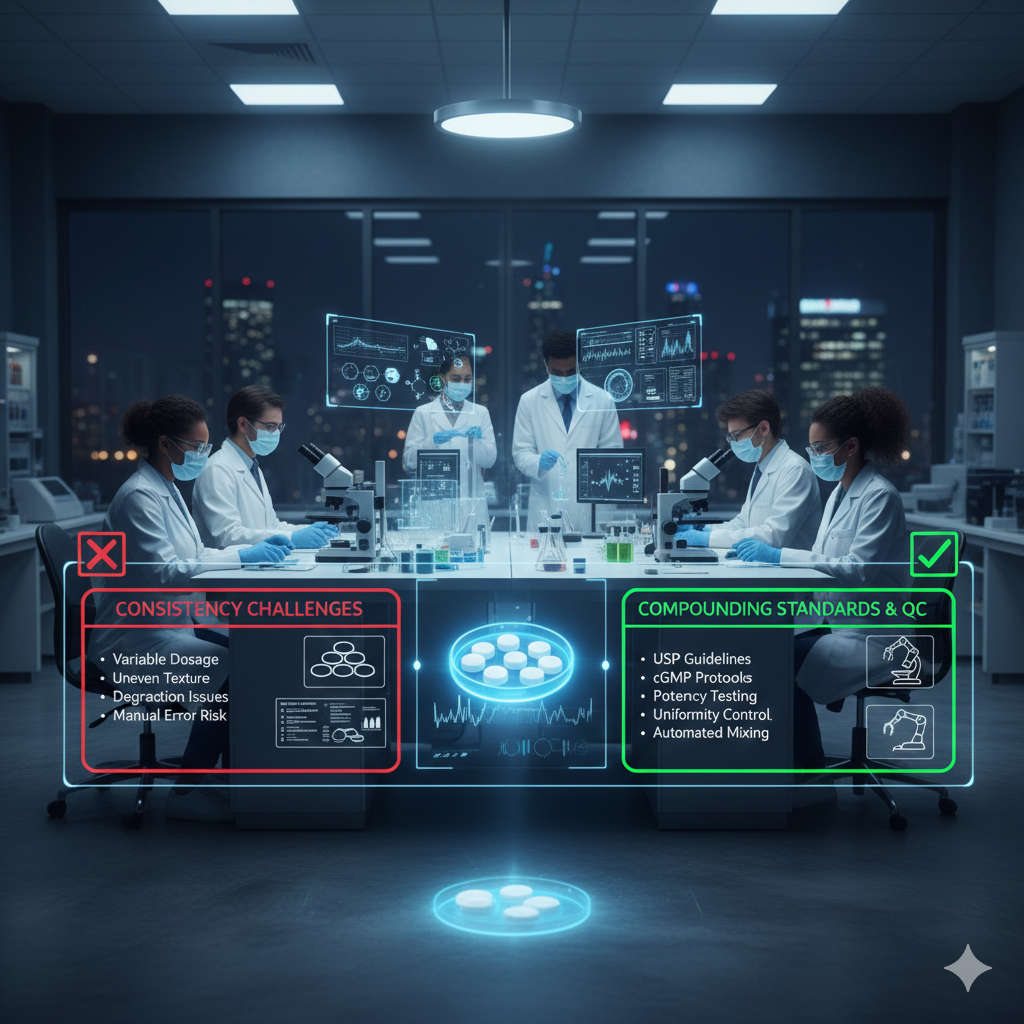
Quality control (QC) is one of the most significant challenges in the compounding of ketamine troches. Pharmacists and compounding pharmacies must conduct multiple tests and checks to verify that each batch of ketamine troches meets the required standards for potency, purity, and consistency.
Potency refers to the amount of ketamine present in each troche. Pharmacists must use accurate instruments to measure and mix the ketamine to ensure that each dose contains the correct amount of the active ingredient. This is particularly important, as variations in potency can significantly affect the efficacy of the treatment.
Purity is equally important in ensuring the safety of the medication. Compounding pharmacies need to verify that the ketamine used is free from contaminants or impurities. This often involves sourcing high-quality ingredients from reputable suppliers and conducting purity tests on the final product.
In addition to potency and purity, pharmacies must also monitor the stability of the troches to ensure they maintain their efficacy over time. Stability testing involves subjecting the compounded troches to different environmental conditions (e.g., temperature, humidity) and assessing any changes in their structure or efficacy.
Batch Testing and Sampling: To further guarantee consistency, pharmacies may implement batch testing and random sampling. Testing a random sample from each batch ensures that the entire batch of ketamine troches meets the required standards, minimizing the risk of discrepancies.
Consistency Challenges in Ketamine Troches
Ensuring consistency in ketamine troches is one of the primary challenges for compounding pharmacies. Even slight variations in the compounding process can lead to inconsistencies in the medication’s effectiveness, which may negatively impact patient outcomes.
- Variation in Ingredients: One of the main factors that can cause inconsistencies is the variation in the quality of excipients used in the troches. Since these ingredients are often sourced from different suppliers, small differences in the formulation can impact the final product’s consistency.
- Compounding Techniques: The skill and experience of the pharmacist compounding the ketamine troches can also play a significant role in the consistency of the final product. Inaccurate weighing or improper mixing can lead to uneven distribution of the active ingredient, leading to suboptimal dosing.
- Environmental Factors: Environmental factors, such as temperature and humidity, can affect the stability and consistency of compounded ketamine troches. Proper storage conditions and adherence to guidelines for handling these products are crucial for maintaining consistency.
- Regulatory Oversight: While the FDA regulates compounding pharmacies, it does not oversee individual compounded products in the same way it does commercially manufactured medications. This means there may be variability in the quality and consistency of ketamine troches produced by different pharmacies.
Conclusion:
As ketamine troches continue to gain popularity as an alternative form of treatment for mental health conditions, it’s essential that pharmacies adhere to the highest compounding standards to ensure quality, consistency, and patient safety. By addressing the challenges related to compounding, quality control, and consistency, healthcare providers and compounding pharmacies can ensure that patients receive safe and effective treatments.
Patients must also be mindful of the need to work closely with their healthcare providers and compounding pharmacists to ensure they are receiving the correct dosage and that the product is being properly prepared and stored. As the use of ketamine troches becomes more common, ongoing research and improvements in compounding practices will be essential to maximizing the benefits of this innovative treatment option.